In the previous article, we introduced the innovative technology of CytScop®Pro. Now, let me take you to uncover the exceptional performance of CytScop®Pro.
Exceptional Performance Chapter
01 Exceptional Performance of Product
When it comes to cell counting instruments, determining the accuracy of the measurement method is crucial. Here, we refer to the cell counting process quality assessment method provided by the ISO 20391-1 Biotechnology - Cell Counting - Part 2: Experimental Design and Statistical Analysis to Quantify Counting Method Performance. We use a dilution series experiment to evaluate the measurement process quality of the CytScop®Pro cell counter.
We randomly selected 3 CytScop®Pro instruments and evaluated their cell counting quality using the experimental design methods and data analysis methods provided by ISO 20391-2. Among them, the Proportionality Index (PI) is used to quantify the degree of conformity of the cell counting method with the proportionality principle. The smaller the PI value, the closer the instrument's test is to the proportionality principle, indicating higher instrument accuracy. Additionally, we also conducted viability analysis to determine the effect of the instrument's measurement process on cell viability.
Stability & Accuracy
The following table shows the CV and PI values of the cell concentration measurements obtained from three CytScop®Pro instruments. Within the optimal concentration measurement range, the average CV is within 3%, indicating that CytScop®Pro possesses excellent stability and accuracy. The small PI values suggest that CytScop®Pro's counting of cell samples conforms more closely to the proportionality principle, demonstrating better adaptability for testing cell samples.
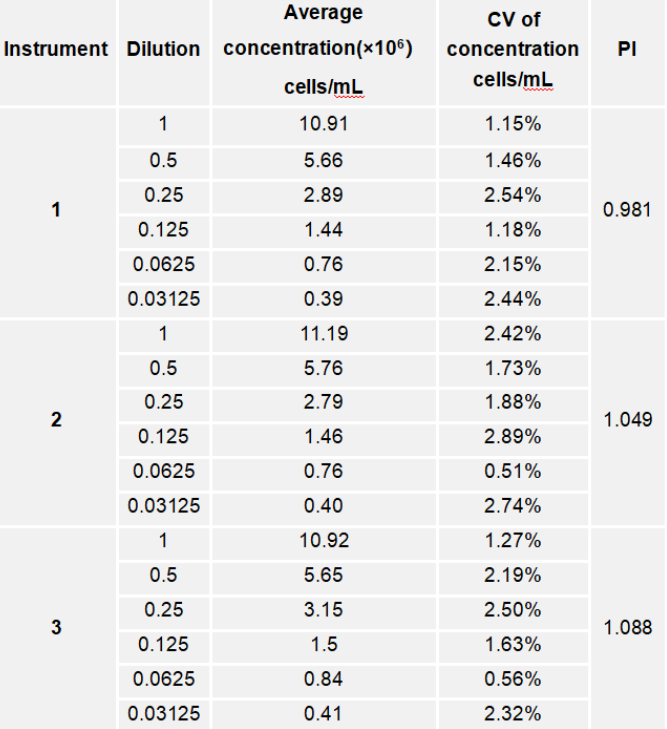
Table 1: Measurement results of various dilution concentrations across the three instruments.
Linearity
The measurement range that fully satisfies accuracy, precision, and good linearity under certain measurement conditions is generally referred to as the Best Range. Within the Best Range, when sampling is performed according to specifications, the R2 value of linear regression is greater than 0.999, and the average CV of repeated samples is less than 3%, demonstrating extremely high stability and consistency.
We have not only conducted linearity studies on a single device under different measurement modes, but also conducted linearity research on three devices.

Figure 1: Linearity Analysis of CytScop®Pro in TB Measurement Mode
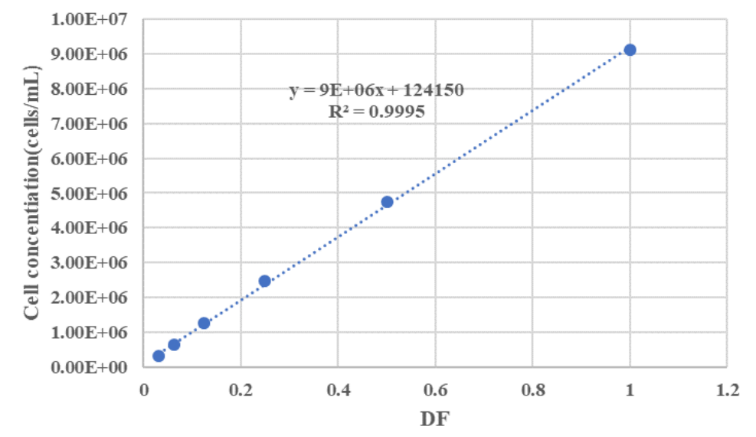
Figure 2: Linearity Analysis of CytScop®Pro in AOPI Measurement Mode
The linear regression analysis conducted on the three instruments showed that the measurement R2 values were all greater than 0.999. The average overall linear correlation analysis of the three instruments resulted in an R2 value of 0.9997. This indicates that CytScop®Pro exhibits excellent linear correlation during the cell counting process, which also suggests that the analytical method possesses good accuracy.
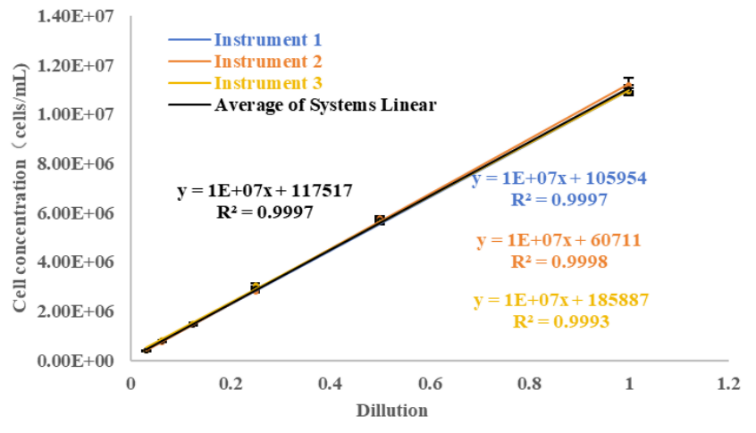
Figure 3: Linear Correlation of Three Instruments under Serial Dilution
Viability
Under each concentration gradient, we analyzed the viability measurements taken by the three CytScop®Pro instruments, and the results are shown in the following figure. It can be seen that under different dilution concentrations, the instrument's measurements have a minimal effect on the viability of cells at different concentrations, with a viability change of no more than 3% compared to the initial state.

Figure 4: Comparison of Cell Viability Measurements by Three Instruments at Various Dilution Concentrations
In summary, CytScop®Pro exhibits excellent accuracy, precision, and linearity during the cell counting process, and has no significant impact on cell viability during measurements.
02 Software Compliance
The CytScop® Pro operates with a 12.1-inch display and its built-in software possesses comprehensive data integrity and regulatory compliance. The software design adheres to the Good Manufacturing Practice (GMP) of the China National Medical Products Administration as well as the rules of the US Food and Drug Administration (FDA) regarding electronic records and electronic signatures (meeting the requirements of 21CFR Part 11), following the ALCOA+CCEA principles.
Standard microspheres of different concentrations used as quality control samples meet the installation and validation requirements for the IQOQ of the CytScop® Pro, providing the utmost safety assurance for its reliable use.
In the next issue, we will delve into the revealing series with you - an in-depth look at the measurement details of CytScop® Pro. Stay tuned for more exciting insights!